Abts Extinction Coefficient 420 Nm. L umol of ABTS per min at 20 C in 01 mol-L phosphate buffer pH 60 using a concentration of 1 rnmol-L ABTS and 01 mmol-L hydrogen peroxide in the reaction mixture. ABTS is best and reliable method for Laccase activity at 420nm ABTS concentration of the mixture should be 02 mM. Percent is as follows. Molar and percent extinction coefficient ε.

Obtain the ΔA 405 nm minute for the maximum linear rate for both the Test and Blank. Molar and percent extinction coefficient ε. Mc is the molar concentration. The enzyme Unit was calculated using molar extinction coefficient value of 36000 M-1 cm-1 for ABTS at 420 nm. ABTS is best and reliable method for Laccase activity at 420nm ABTS concentration of the mixture should be 02 mM. Where E is the extinction coefficient.
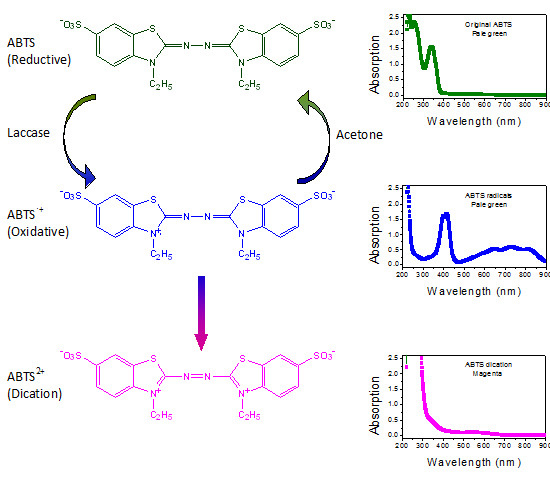
Absorbance was read at 420 nm in a spectrophotometer against a suitable blank Sunil et al 2011.
RB19 595 nm and K4FeCN 6 420 nm ε is the extinction coefficient of the substrate oxidation product ABTS 293 mM -1 cm -1 VA 93 mM -1 cm -1 26-DMP 273 mM -1 cm -1 guaiacol 557 mM -1 cm -1 RB19 10 mM -1 cm -1 and K. Both at pH 60 and 44 the activity expressed in UmL-1 was. E A mc. This inequality is fully compatible with the accepted value of the extinction coefficient of the radical at 734 nm 15 x 10 4 M-1 cm-1 and indicates that the high stoichiometric factors obtained for some compounds are not due to an overestimation of the radical cation concentration. 516 and 744 nm in DPPH and ABTS assays respectively. A is the absorbance.