Determination Of Cmc Of Surfactants. This is followed by the detection of some characteristic point - which is called the CMC. The change of slope or dis-continuity of κc dependence gives the CMC. In this review methodological approaches to the determination of the CMC of surfactants by CE technique are described. The CMC values obtained closely agree with those determined by other methods including measurements of static.
The CMC values obtained closely agree with those determined by other methods including measurements of static. The nonionic surfactants form donoracceptor complexes with iodine in aqueous medium. Critical micelle concentrations have been experimentally determined using a number of different methodologies. This is followed by the detection of some characteristic point - which is called the CMC. The change of slope or dis-continuity of κc dependence gives the CMC. The formation of micellar aggregates causes significant changes in a number of physical and chemical properties of the system such as surface tension conductivity fluorescence pH etc.
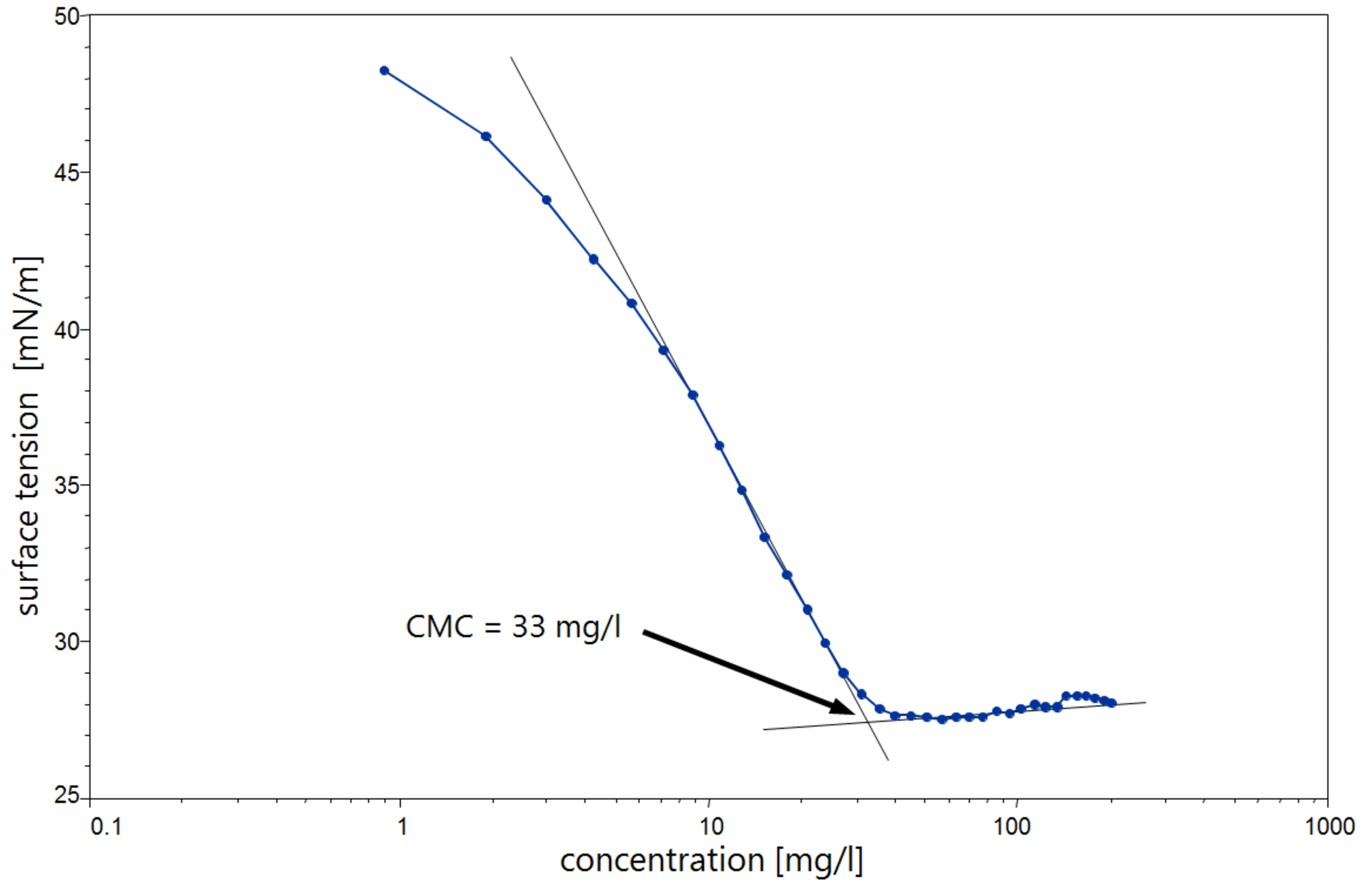
The data corresponding to the chosen physical property obtained through the corresponding methodtechnique usually fits into two straight lines with different slopes for the pre- and postmicellar concentrations.
The laboratory experiments described in this work present the CMC-determination of some surfactants by following three different methods which require the use of the very common techniques in physical chemistry laboratories such as UV-Vis spectroscopy luminescence spectroscopy and electrical conductivityIn performing these experiments the CMC of a surfactant is determined by measuring a. To check the reliability of the recom-mended CE method the dye solubilization and the con-ductometric methods described in experimental section were also carried out. Experimentally the cmc of any surfactant is determined graphically by plotting the magnitudevariations of an appropriate physical property as a function of surfactant concentration. The spectral absorption and the shift in the λ max of l 2 upon complexation have been exploited to determine the critical micelle concentration CMC of Tweens Brijs and Triton X100. The formation of micellar aggregates causes significant changes in a number of physical and chemical properties of the system such as surface tension conductivity fluorescence pH etc. Determination of Critical Micelle Concentration CMC of a Surfactant.