Electron Configuration Of Ferrous Ion. Click hereto get an answer to your question Electronic confirguration of ferrous ion is. IRON IONFe 2 UNII-GW89581OWR. Iron II Fe 2 ion. When ferrous ions are present in water it gives a clear colourless solution.

For Fe n4 N shell In Iron the electronic. We also discuss Electronic configuration of ferrous Electronic configuration of ferric E. This is an important component of the toxicity of ferrous iron. The Ferric Ion is more stable than the Ferrous Ion. Basic iron happens in a low-oxygen condition despite the fact that it is receptive to water and oxygen. The electron configuration of F is 1s2 2s2 2p5.
IRON IONFe 2 UNII-GW89581OWR.
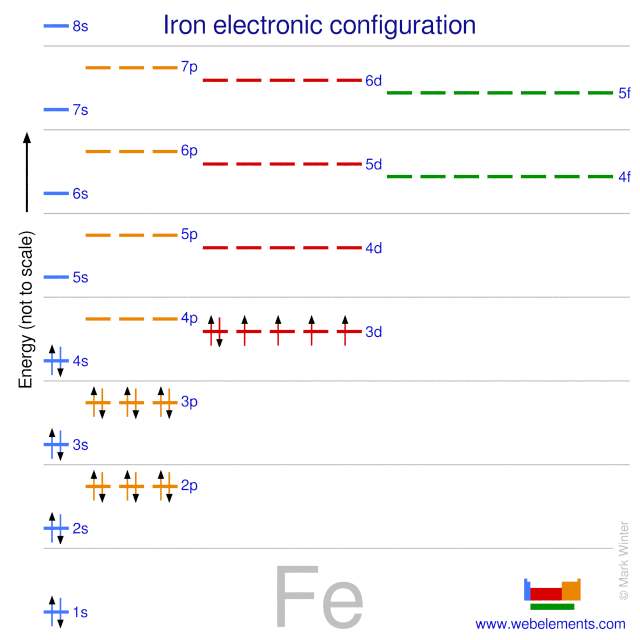
In Ferric ion there are 5 electrons in d orbitals so d is half filled which is stable electronic configuration in Ferrous ion d orbitals have 6 electrons. And for ferrous ion 24 electrons specified as requiredwhy as required. Denoting the charged state Equivalent notations for an iron atom Fe that lost two electrons referred to as ferrous. 1s22s22p63s23p64s23d6 for atomic iron26 electrons specified as required. The electron configuration for Fe2 is 1s2 2s2 2p6 3s2 3p6 3d6The electron configuration for Fe3 is 1s2 2s2 2p6 3s2 3p6 3d5Ask me questions. Therefore ferric ions are relatively stable than ferrous ions.