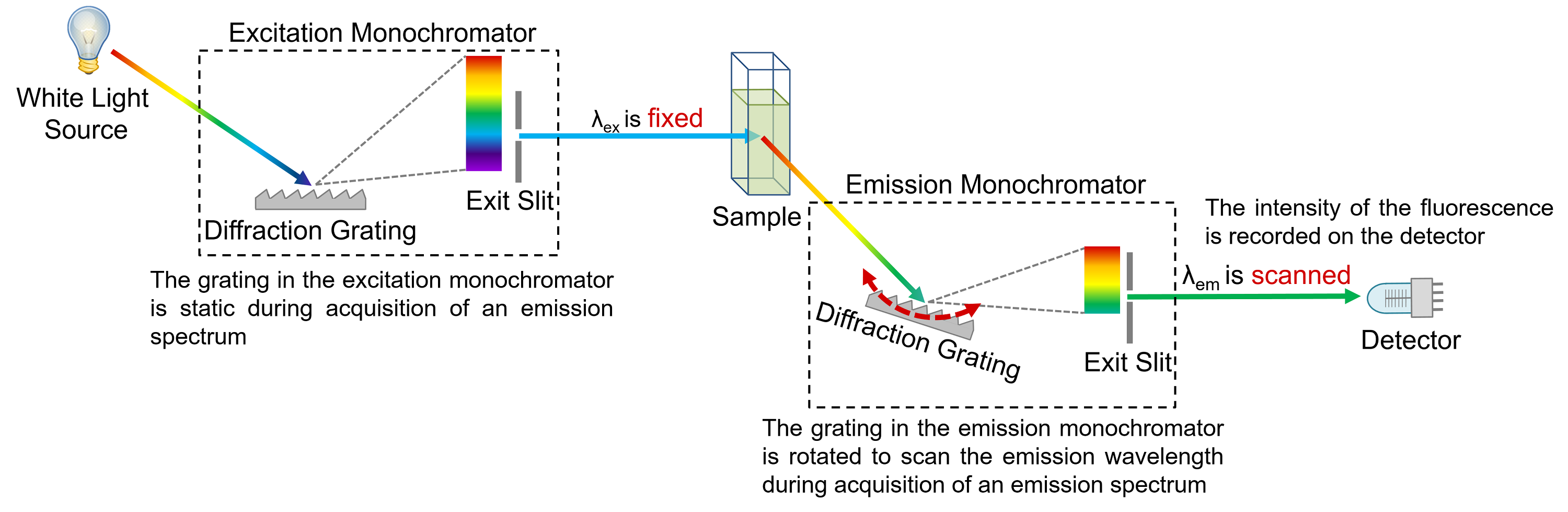
Excitation And Emission Fluorescence. The excitation wavelength was 805. The emission maximum is chosen and only emission light at that wavelength is allowed to pass to the detector. Because of their novel electronic configurations fluorochromes have unique and characteristic spectra for absorption usually similar to excitation and emission. B Fluorescence emission spectra from an ensemble red and three different individual FMO complexes black.

This phenomenon makes it closer to absorption spectrum rather thanfluorescence emission spectrum. They are intended to provide a reference source that relates the spectral properties of these fluorochromes to commercially available. The emission maximum is chosen and only emission light at that wavelength is allowed to pass to the detector. These curves describe the likelihood that excitation and emission will occur as a function of wavelength and provide important information about the expected behavior of the irradiated fluorophore. In the fluorescence excitation spectrum the emission intensity is proportional to the absorbance of the molecule. The fluorescence excitation spectrum characterizes the electron distribution of the molecule in the ground state.
The basic fluorescence properties of a fluorophoreexcitation and emissionare often presented in the form of line graphs.
Fluorescence was determined using either a 48520 excitation 53025 emission filter set or a 40030 excitation 50820 emission filter set. The excitation wavelength was 805. The three-stage process of excitation excited lifetime and emission is called fluorescence. The excitation spectrum of a given fluorochrome is determined in a similar manner by monitoring fluorescence emission at the wavelength of maximum intensity while the fluorophore is excited through a group consecutive wavelengths. Excitation maxima and emission maxima. Fluorescence Excitation and Emission Fundamentals Fluorescence is a member of the ubiquitous luminescence family of processes in which susceptible molecules emit light from electronically excited states created by either a physical for example absorption of light mechanical friction or chemical mechanism.