
Free Co2 In Water Sample. The reaction between water and dissolved carbon dioxide is reversible and rapid. We get a CO2 reading in that water of 320ppm. B Persulphate Heat or UV oxidation Organic carbon is released as carbon dioxide from a solution of persulphate into which the sample has been introduced. TOC may also refer to the amount of organic carbon in soil or in a geological formation particularly the source rock for a petroleum play.
We have to subtract the SAME atmospheric Co2 from the Ocean Co2-320-330 is -10 on the chart. B Persulphate Heat or UV oxidation Organic carbon is released as carbon dioxide from a solution of persulphate into which the sample has been introduced. Surface water normally contains less than 10 ppm of free carbon dioxide while some ground waters may exceed that concentration. Titrant used was N a O H and analyte was sample water. Then we sample SS water that is 20C. C O X 2 g C O X 2 a q 1 C O X 2 a q H X 2 O H X 2 C O X 3 H X H C O X 3 X.
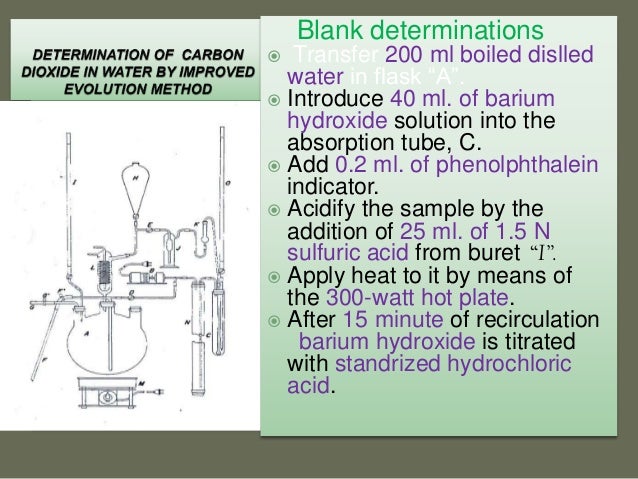
Classical method Buret titration method.
The electron rich oxygen of water donates an electron pair to the carbon. The carbon atom of CO 2 is electron poor with an oxidation state of IV. Instrumental method Coulometry method. This method involves purging an acidified sample with carbon-free air or nitrogen prior to measurement and so is more accurately called non-purgeable organic carbon NPOC. The electron rich oxygen of water donates an electron pair to the carbon. Total organic carbon TOC is the amount of carbon found in an organic compound and is often used as a non-specific indicator of water quality or cleanliness of pharmaceutical manufacturing equipment.