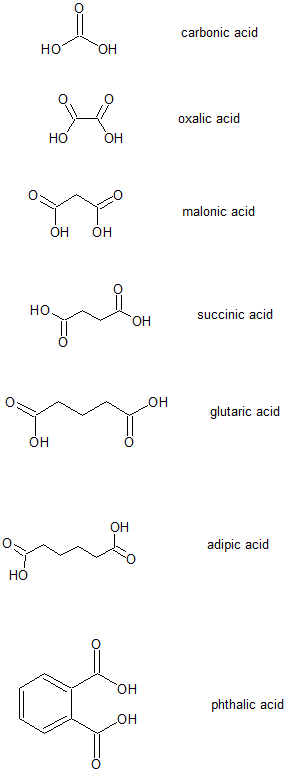
Functional Group Of Oxalic Acid. These oxalates can form larger kidney stones that can obstruct the kidney tubules. Wholesale Price 3 hydroxy 2 naphthoic acid solubility - NN-Diethylaniline 91-66-7 professional manufacturer EINECS No. But this is not necessarily the final grouping as functional groups may be added in between to ensure all groups are listed alphabetically The secondary functional groups are. Formic acid and acetic acid are the simplest aliphatic acid and benzoic acid is the simplest aromatic acid.
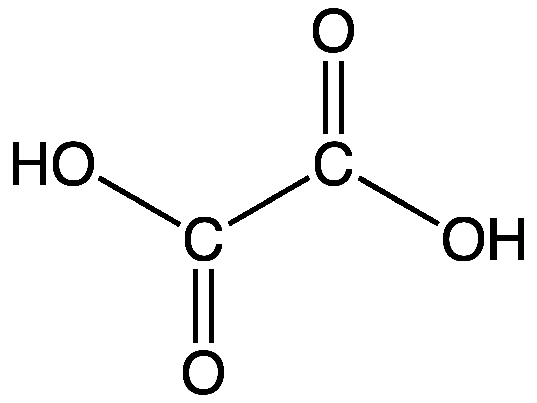
The CO functional group is present in aldehydes and ketones. Oxalic acid is the only possible compound in which two carboxyl groups carboxyl group in chemistry functional group that consists of a carbon atom joined to an oxygen atom by a double bond and to a hydroxyl group OH by a single bond. Carboxylic acids are compounds whose molecules contain a carboxyl group that is joined to a hydrogen atom an. Advertentie Quality Chemicals For Research Used Only. Advertentie Quality Chemicals For Research Used Only. Ketones contain the carbonyl inside the compound and aldehydes contain the carbonyl at the end of the organic compound.
An αω-dicarboxylic acid that is ethane substituted by carboxyl groups at positions 1 and 2.
Depending on the location of the carbonyl group it is termed differently. Esters have a pair of alkyl or aromatic groups attached to a carbonyl linking oxygen function. Carboxylic acids are compounds whose molecules contain a carboxyl group that is joined to a hydrogen atom an. The carbonyl functional group is a carbon double bonded to an oxygen. The side chains are grouped like this. Carboxylic acids are organic compounds containing carboxyl functional group.