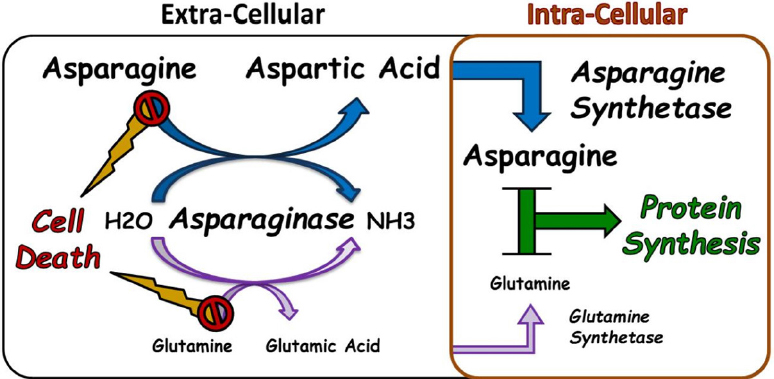
L Asparaginase A Review. Complete asparagine depletion is observed less frequently with enzyme concentrations below this level4344 However there is some evidence to suggest that trough asparaginase levels of below 50 IUL. L-asparaginase is a critical part of the treatment of acute lymphoblastic leukaemia in children and adolescents and has contributed to the improvement in patient outcomes over the last 40 years. Also this review lists the sources of L-asparaginase and L-glutaminase production optimization of enzymes and uses of the two enzymes in cancer therapy and other industrial purposes. This review hence mainly focuses on the biochemical aspects of L-asparaginase production aiming to comprehend the physiochemical characteristics application and assay methods of L-asparaginase enzyme properties and kinetics of recombinant enzyme production by fermentation.
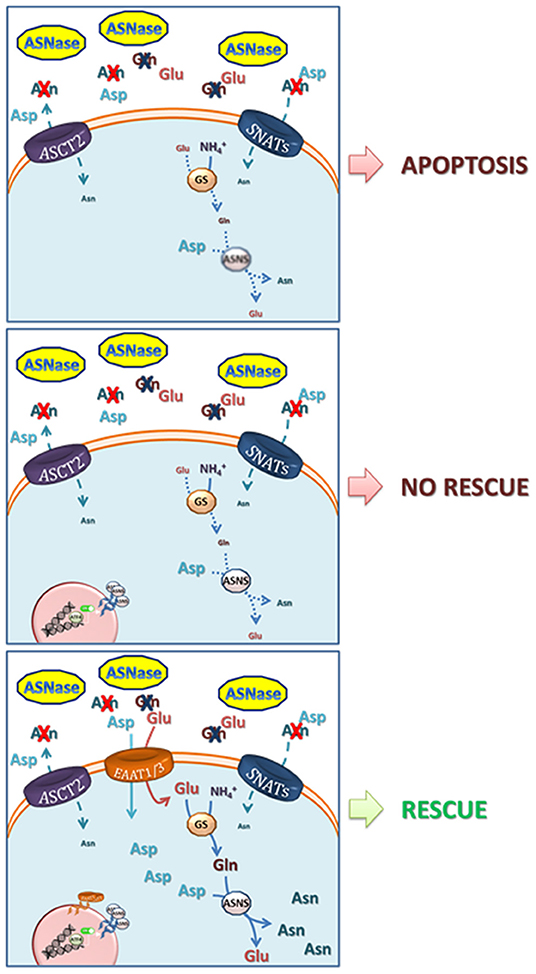
Ulu A1 Ates B1. There are two major applications of l-asparaginase L-ASNase. 3511 also known as L-asparagine amidohydrolase is the enzyme with anti-tumor activity and is well accepted as a chemotherapeutic agent against the acute lymphoblastic leukemia and lymphosarcoma. Leukemia therapy and the food industry. Pieces of information about the three-dimensional structure of L-asparaginase from Escherichia coli and Erwinia sp. Have identified residues that are essential for catalytic activity.
Have identified residues that are essential for catalytic activity.
Leukemia therapy and the food industry. Occurrence of l-asparaginases in fungi yeasts bacteria and animal cells and their antitumour effects were reviewed in many articles 84 85 but the majority of l-asparaginases cannot be. 1Department of Chemistry Faculty of Science Arts Inonu University Malatya 44280 Turkey. The main products used in clinical treatment are L-asparaginase enzymes derived from Escherichia coli and Erwinia chrysanthemi. This study followed the Preferred Reporting Items for Systematic Reviews. This article has briefly.