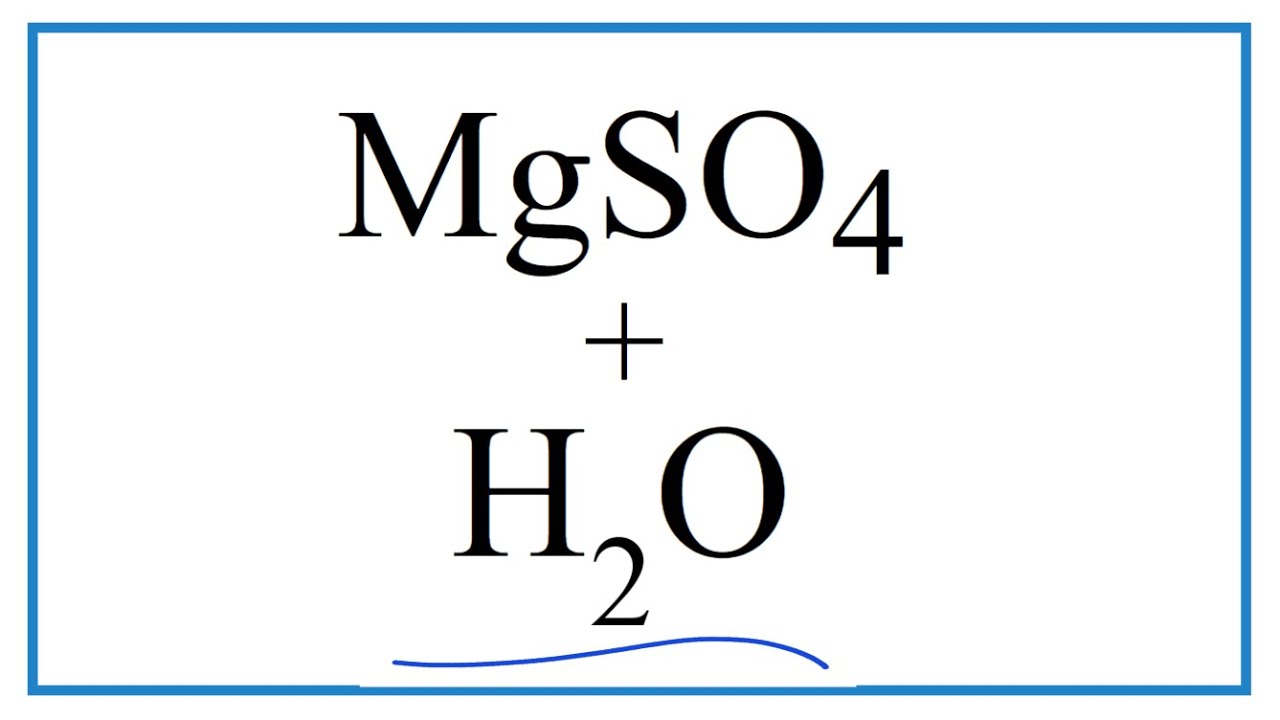
Magnesium Sulfate And Water Equation. Magnesium Sulphate Contribution Pure Water Contribution where the pressure dependencies are given by P 1 P 2 and P 3 and the relaxation frequencies are f 1 and f 2. According to the balanced chemical equation. This reaction evolves heat meaning it is exothermic. Other magnesium compounds are clearly more water soluble for example magnesium carbonate 600 mgL.

This reaction evolves heat meaning it is exothermic. The chemical equation between magnesium sulfate and water can be given as below. When salt dissolves in a solvent the reaction called disolution can be endothermic or exothermic depending on the type of salt. This problem was formulated by Cisternas 1999. H2SO4 MgO — H2O MgSO4 This is a balanced equation. Magnesium Sulphate Contribution Pure Water Contribution where the pressure dependencies are given by P 1 P 2 and P 3 and the relaxation frequencies are f 1 and f 2.
The chemical equation between magnesium sulfate and water can be given as below.
Perry Don Green Seventh Edition. Other magnesium compounds are clearly more water soluble for example magnesium carbonate 600 mgL. In order to determine this we weighed the mass of Magnesium Sulfate boiled it for about 10 minutes until it became an anhydrous salt then found the weight of its anhydrous salt and the water in the hydrate just like the steps we did for the Copper. To explore more about the structure physical and chemical properties of Magnesium sulfate MgSO 4 from the experts register with BYJUS now. First of all Dissolution of periclase magnesium hydroxide or magnesium carbonate in dilute vitriol. The nonmetal retains its oxidation number.