Nucleic Acid Amplification Test Naat. SDA strand displacement assay. PPV positive predictive value. With rapid turnaround time and ease of use NAAT assays can be considered as point-of-care tests for diagnosis of GAS replacing the need for back-up culture. Michael saag director uab infectious diseases division said.
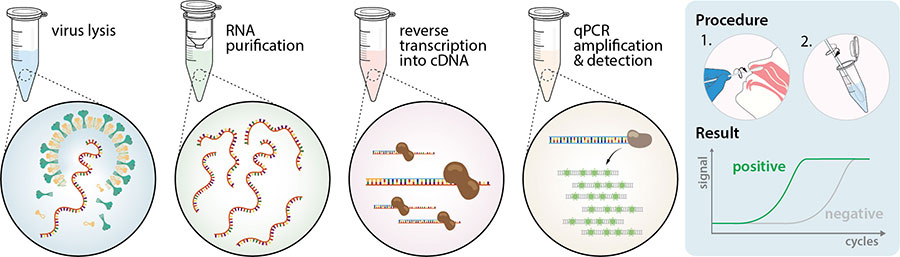
The molecular chemistry of INAAT is more complex than that of RT-PCR involving more primers and intricate conformational. NLR negative likelihood ratio. The latest Centers for Disease Control and Prevention CDC guidelines recommend a Nucleic Acid Amplification Test NAAT a form of molecular testing as the preferred method when testing for trichomoniasis. Notice that the inclusion of amplification in the definition means that any NAAT necessarily involves some kind of PCR procedure but does not imply that the PCR step is where the test happens. NPV negative predictive value. With rapid turnaround time and ease of use NAAT assays can be considered as point-of-care tests for diagnosis of GAS replacing the need for back-up culture.
With rapid turnaround time and ease of use NAAT assays can be considered as point-of-care tests for diagnosis of GAS replacing the need for back-up culture.
Detects but does not differentiate toxin A gene tcdA and toxin B gene tcdB. Testing by nucleic acid amplification tests NAAT. Detects but does not differentiate toxin A gene tcdA and toxin B gene tcdB. Testing for Neisseria gonorrhoeae GC as a single test by Nucleic Acid Amplification is not performed and requests will be tested with the CTGC combo assay. Nucleic acid amplification testing NAAT amplifies and detects nucleic acid sequences that are specific for the organism being detected. FP false positive.