
Oxidation Number Of Sulfur In Sulfate. The reduction of elemental sulfur frequently occurs when oxygen is lacking. They include thiosulfate S 2 O 3 2 with an average sulfur oxidation state of 2 dithionite often as the sodium salt Na 2 S 2 O 4 with an average S oxidation state of 3 sulfur dioxide SO 2 used in wine storage as an antimicrobial agent that has sulfur in the 4 oxidation state. Sulfur has an oxidation number of 6 or it loses 6 atoms when it forms a chemical compound. Sulfur number 16 has oxidation numbers of -2 2 4 and 6.
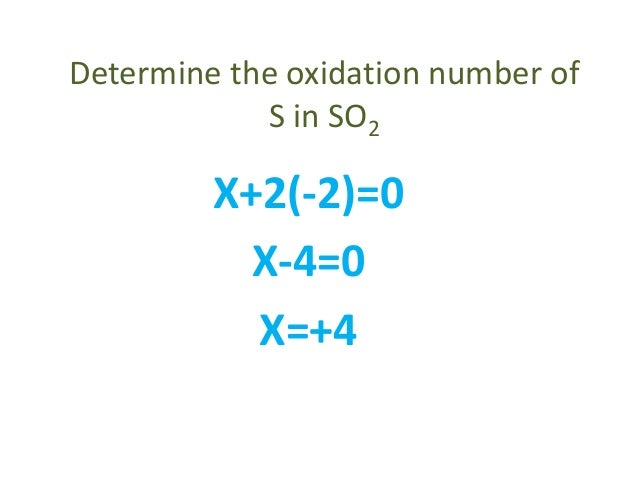
S2O82- is 2S 6-2 2-1 -2 2S -12 S -6. Now if we just solve this equation will have X Um plus to minus. That means minus two times for and this total sum will be called to the net charge on the compound. Of course in sulfates SO_42- and sulfites SO_32- the sulfur atom assumes formal oxidation states of VI and IV. As you know oxidation states are assigned per atom so you can say that 1 xx ON_sulfur 4 xx ON_oxygen -2 This means that the oxidation state of sulfur is ON_sulfur 4 -2 -2 ON_sulfur -2 8 colorgreen6 The oxidation states for this compound are stackrelcolorblue1Na_2stackrelcolorblue6S. In this case the overall charge of the sulfate anion is 2-.
The -ate ending indicates that the sulfur is in a negative ion.
Now if we just solve this equation will have X Um plus to minus. Fluorine in compounds is always assigned an oxidation number of -1. The reduction of elemental sulfur frequently occurs when oxygen is lacking. The residence time of aerosol sulfur of anthropogenic origin and the mean-life time of sulfur dioxide for the oxidation are estimated to be 10 days and 30 hrs respectively from the concentrations of sulfate and 210Pb in precipitation. The oxidation of elemental sulfur S⁰ to sulfate sulfur SO₄ in soil is a biological process and is carried out by several kinds of microorganisms. Of course in sulfates SO_42- and sulfites SO_32- the sulfur atom assumes formal oxidation states of VI and IV.