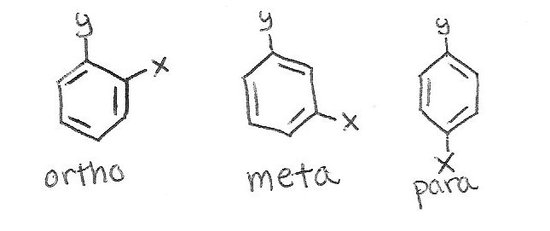
Para Position In Benzene Ring. Form representing pará preposition beside alongside of by beyond para- 2. The terms ortho meta and para are prefixes used in organic chemistry to indicate the position of non-hydrogen substituents on a hydrocarbon ring benzene derivative. There are three relative positions for a disubstituted benzene ring. Rings through the ortho positions of benzene are very readily formed and offer a striking contrast to the comparatively rare meta and para rings.

The activating group directs the reaction to the ortho or para position which means the electrophile substitute the hydrogen that is on carbon 2 or carbon 4. The assumption that meta and para ring structures exist in certain com pounds has been made occasionally but only in a few instances has the existence of such structures been proved with certainty. The terms ortho para and meta refer to different structures of a benzene ring with at least two substituents. Ortho meta and para. The key difference between ortho para and meta substitution is that ortho substitution has two substituents in 1 and 2 positions of the ring and. Before a vowel par-.
All the functional groups are divided into ortho - para or meta -directors.
The terms ortho meta and para are prefixes used in organic chemistry to indicate the position of non-hydrogen substituents on a hydrocarbon ring benzene derivative. The key difference between ortho para and meta substitution is that ortho substitution has two substituents in 1 and 2 positions of the ring and. They are defined as the following. Remember when assigning the position of a new substituent to a benzene ring activating groups always dictate the position of the new substituent. Para position in organic chemistry is the one in which there are two same functional groups tied to a ring of benzene in the position 1 and 4. We know that activating groups direct new substituents to the ortho or para positions.