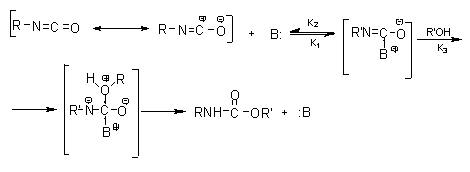
Reaction Of Isocyanate With Alcohol. For example they can react with compounds containing active hydrogen atoms like amines alcohols mercaptanes water and carboxylic acids or they can react with themselves to form dimers. The system most exhaustively studied was the reaction of a-naphthyl isocyanate with n-butyl alcohol using ferric acetylacetonate as catalyst and diethylene glycol diethyl ether as solvent. There is another field in which the reaction of isocyanates in excess with trace levels. Based on the kinetic results it was found that i 26TDI reacts faster with alcohols.

Initial slopes for the reaction at several. The kinetics of the reaction between 26toluene diisocyanate 26TDI and alcohols with varying carbon chain lengths and a primary or secondary hydroxyl group in the absence and presence of tinII 2ethylhexanoate TEH as a catalyst were studied. This review focuses on catalysis the mechanism involved in the formation of Non-Isocyanate Polyurethanes NIPU from five-membered cyclic carbonates and the reaction. The objective of this work was to obtain primary amine groups on the surface of polyvinyl alcohol films by means of a reaction with hexamethylene diisocyanate. Reactivity is influenced by structure and primary secondary and tertiary hydroxyls have decreasing reactivity due to neighboring methyl. Catalyse the isocyanate-hydroxyl reaction by complex formation with both isocyanate and hydroxyl groups.
The system most exhaustively studied was the reaction of a-naphthyl isocyanate with n-butyl alcohol using ferric acetylacetonate as catalyst and diethylene glycol diethyl ether as solvent.
The structure of the resulting polymers was determined by means of IR 1 H and 13 CNMR spectroscopy as well as by chemical analysis. The objective of this work was to obtain primary amine groups on the surface of polyvinyl alcohol films by means of a reaction with hexamethylene diisocyanate. New insight into the kinetics of diisocyanate-alcohol reactions by high-performance liquid chromatography and mass spectrometry. An isocyanate excess is generally not used. Catalyse the isocyanate-hydroxyl reaction by complex formation with both isocyanate and hydroxyl groups. Di ff erent metal based catalysts Edelmann concluded.