Tempo Oxidation Of Primary Alcohol To Aldehyde. The NaIO 4 TEMPONaBr system provides a mild and efficient method for the oxidation of alcohols that are sensitive to basic conditions. As an example copper II complexes combined with a stable nitroxyl radical such as 2266-tetramethylpiperdine-N-oxyl TEMPO molecular oxygen and K2 CO 3 enable the selectively aerobic oxidation of primary benzylic alcohols to their corresponding aldehydes in alkaline water solution. 2266-Tetramethyl-1-piperidinyloxy catalyzes efficient oxidation of primary alcohols to aldehydes by N-chlorosuccinimide in a biphasic dichloromethaneaqueous pH 86 buffer system in the presence of tetrabutylammonium chloride. The ionic liquid can be reused after washing with.

A practical general and mild oxidation of primary and secondary alcohols to carbonyl compounds proceeds in yields of up to 99 using SO 2 F 2 as electrophile in DMSO as both the oxidant and the solvent at ambient temperature. The NaIO 4 TEMPONaBr system provides a mild and efficient method for the oxidation of alcohols that are sensitive to basic conditions. The resulting aldehydes with no traces of carboxylic acids and ketones can be extracted with organic solvents. Regardless the high activity towards benzylic alcohols the challenge for the copper II based. The product can be isolated by a simple extraction with organic solvent and the ionic liquid can be recycled or reused. A TEMPO-catalyzed selective oxidation of alcohols to the corresponding aldehydes and ketones using NaIO 4 as the terminal oxidant is reported.
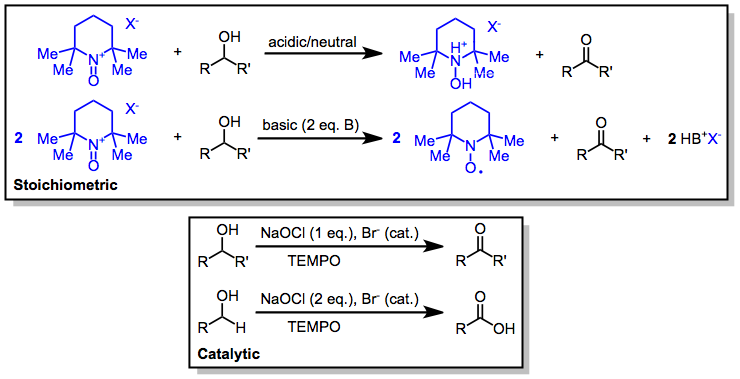
The oxidation of an alcohol to form an aldehyde or ketone is very important in synthesis.
Secondary alcohols are oxidized to ketones with a much lower. The catalyst system is compatible with a wide range of functional groups and shows a high selectivity for 1 alcohols. A simple and mild TEMPOCuCl catalyzed aerobic oxidation of primary and secondary alcohols to the corresponding aldehydes and ketones in ionic liquid bmimPF6 with no trace of overoxidation to carboxylic acids has been developed. This protocol describes a practical laboratory-scale method for aerobic oxidation of primary alcohols to aldehydes using a chemoselective CuITEMPO TEMPO 2266-tetramethyl-1-piperidinyloxyl catalyst system. A bpyCuITEMPO catalyst system enables an efficient and selective aerobic oxidation of a broad range of primary alcohols including allylic benzylic and aliphatic derivatives to the corresponding aldehydes using readily available reagents at room temperature with ambient air as the oxidant. Use of air-microbubbles to improve gas absorption into liquid phase is proven to be highly beneficial for gasliquid phase reactions.