
What Is Infrared Absorption Spectroscopy. Characterizing the Spoilage of Egg Products using Targeted and Non-targeted Approaches. It covers a range of techniques mostly based on absorption spectroscopy. This analysis method is used to analyze organic compounds to determine what kind of functional groups substituents are included in the compounds. At selected wavelengths however the radiations intensity is attenuated.
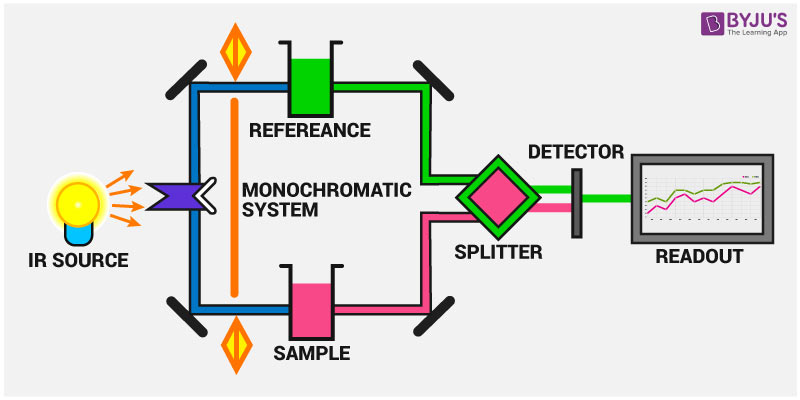
CO2 N20 and Halogenated agent monitoring eg everything apart from oxygen operates around the absorption spectroscopy principle specifically absorption of infrared light. Infrared Spectroscopy is the analysis of infrared light interacting with a molecule. Infra-red radiation also consists of a continuous range of frequencies - it so happens that our eyes cant detect them. This technique covers the region of the electromagnetic spectrum between the visible wavelength of 800 nanometres and the short-wavelength microwave 03 millimetre. In absorption spectroscopy a beam of electromagnetic radiation passes through a sample. This can be analyzed in three ways by measuring absorption emission and reflection.
Infrared spectroscopy IR spectroscopy is the spectroscopy that deals with the infrared region of the electromagnetic spectrum that is light with a longer wavelength and lower frequency than visible light.
Infrared spectrometers similar in principle to the UV-Visible spectrometer described elsewhere permit chemists to obtain absorption spectra of compounds that are a unique reflection of their molecular structure. One of these analysis methods is infrared spectroscopy. Much of the radiation passes through the sample without a loss in intensity. You probably know that visible light is made up of a continuous range of different electromagnetic frequencies - each frequency can be seen as a different colour. It is used by. Infrared absorption also known as infrared spectroscopy is a type of study that is used to identify and study chemicals.